Is autism an unintended consequence of attempts to reduce meningitis?
Conjugate vaccines under suspicion
There is a brilliant hypothesis by Dr. Brian J. Richmand from 2011 that has not received the deserved attention and has not been refuted:
“The first conjugate vaccine was approved for use in the US in 1988 to protect infants and young children against the capsular bacteria Haemophilus influenzae type b (Hib). Since its introduction in the US, this vaccine has been approved in most developed countries, including Denmark and Israel where the vaccine was added to their national vaccine programs in 1993 and 1994, respectively. There have been marked increases in the reported prevalence of autism spectrum disorders (ASDs) among children in the US beginning with birth cohorts in the late 1980s and in Denmark and Israel starting approximately 4-5 years later. Although these increases may partly reflect ascertainment biases, an exogenous trigger could explain a significant portion of the reported increases in ASDs. It is hypothesized here that the introduction of the Hib conjugate vaccine in the US in 1988 and its subsequent introduction in Denmark and Israel could explain a substantial portion of the initial increases in ASDs in those countries. The continuation of the trend toward increased rates of ASDs could be further explained by increased usage of the vaccine, a change in 1990 in the recommended age of vaccination in the US from 15 to 2 months, increased immunogenicity of the vaccine through changes in its carrier protein, and the subsequent introduction of the conjugate vaccine for Streptococcus pneumoniae.
Although conjugate vaccines have been highly effective in protecting infants and young children from the significant morbidity and mortality caused by Hib and S. pneumoniae, the potential effects of conjugate vaccines on neural development merit close examination. Conjugate vaccines fundamentally change the manner in which the immune systems of infants and young children function by deviating their immune responses to the targeted carbohydrate antigens from a state of hypo-responsiveness to a robust B2 B cell mediated response. This period of hypo-responsiveness to carbohydrate antigens coincides with the intense myelination process in infants and young children, and conjugate vaccines may have disrupted evolutionary forces that favored early brain development over the need to protect infants and young children from capsular bacteria”.
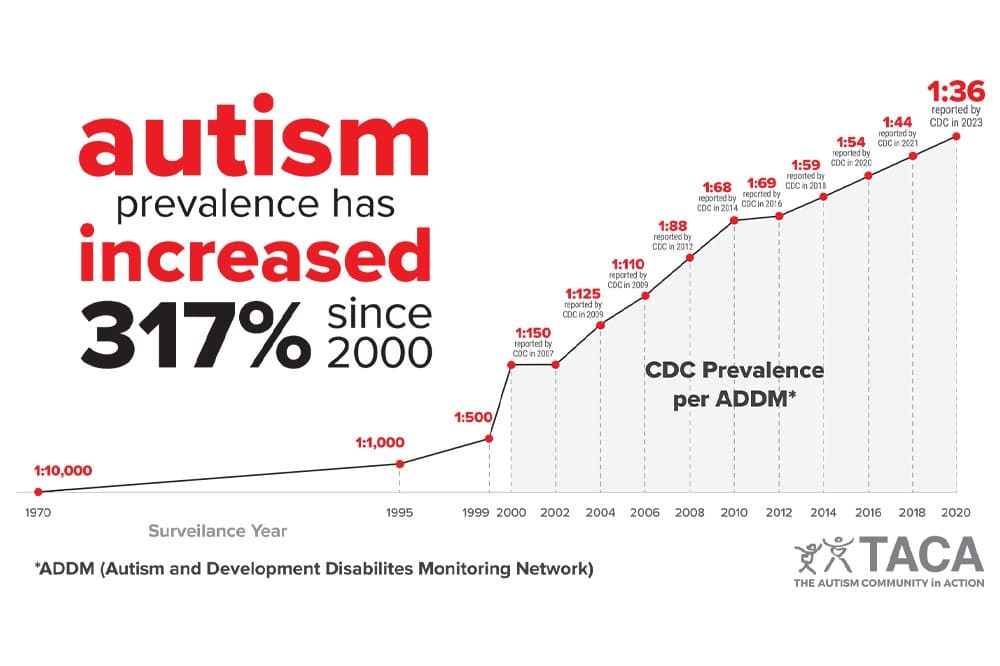
And now Sally Beck, TACA and the CDC present chilling data:
It is timely to review this:
Chronology of infant vaccination against meningitis with capsular polysaccharides
Vaccines against Haemophilus influenzae Type b (Hib):
1987: ProHIBit: first conjugate vaccine against Hib, using diphtheria toxin as a carrier protein. Indicated for primary vaccination.
1989: HibTITER: conjugate vaccine with CRM197, a non-toxic variant of diphtheria toxin. Indicated for primary vaccination.
1993: PedvaxHIB: conjugate vaccine with outer membrane protein complex (OMPC) from Neisseria meningitidis. Indicated for primary vaccination. With aluminum.
1993: ActHIB: conjugate vaccine with tetanus toxoid, indicated for primary vaccination.
1996: Hiberix: conjugate vaccine with tetanus toxoid, initially licensed only as a booster dose (Europe).
2009: Hiberix: FDA approves Hiberix for use as a booster dose in children aged 15 months through 4 years.
2016: Hiberix: FDA expands Hiberix's approval to include its use as a primary vaccination series for infants, administered at 2, 4, and 6 months of age.
Vaccines against Streptococcus pneumoniae (Pneumococcus):
2000: Prevnar (PCV7): first conjugate vaccine against 7 pneumococcal serotypes, using CRM197 as a carrier protein. Indicated for infants and young children. With aluminum.
2010: Prevnar 13 (PCV13): replaced PCV7, expanding coverage to 13 pneumococcal serotypes. With aluminum.
Vaccines against Neisseria meningitidis (Meningococcus):
1999: Menjugate: conjugate vaccine against meningococcus C, with CRM197 toxin. With aluminum.
2010: Menveo: conjugate vaccine against serogroups A, C, W, and Y, using CRM197 toxin.
2010: Nimenrix: conjugate vaccine against serogroups A, C, W, and Y, using tetanus toxoid.
2015: Bexsero: vaccine against meningococcus B, based on bacterial surface proteins. With aluminum.
So let's now take another look at the growing autism graph and think: